Following Excitation/Inhibition Ratio Homeostasis from Synapse to EEG in Monogenetic Neurodevelopmental Disorders
Lisa Geertjens, Torben W. van Voorst, Arianne Bouman, Maaike A. van Boven, Tjitske Kleefstra, Matthijs Verhage, Klaus Linkenkaer-Hansen, Nael Nadif Kasri, L. Niels Cornelisse, Hilgo Bruining
Genes 2022, 13, 390
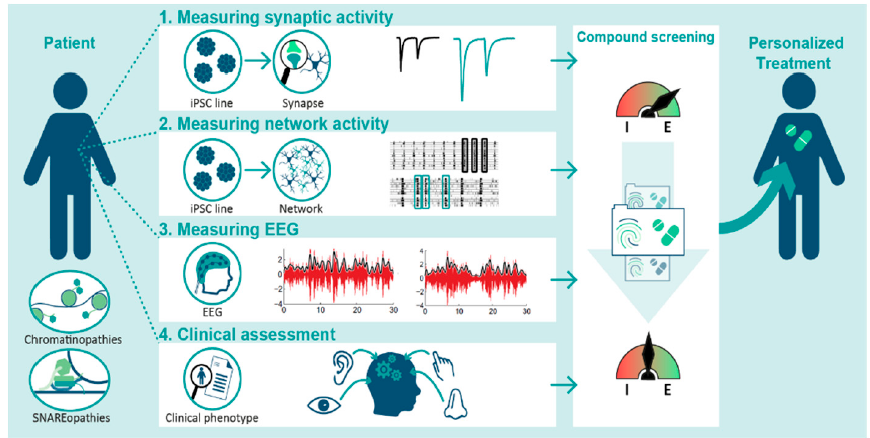
Pharmacological options for neurodevelopmental disorders are limited to symptom suppressing agents that do not target underlying pathophysiological mechanisms. Studies on specific genetic disorders causing neurodevelopmental disorders have elucidated pathophysiological mechanisms to develop more rational treatments. Here, we present our concerted multi-level strategy ‘BRAINMODEL’, focusing on excitation/inhibition ratio homeostasis across different levels of neuroscientific interrogation. The aim is to develop personalized treatment strategies by linking iPSC-based models and novel EEG measurements to patient report outcome measures in individual patients. We focus our strategy on chromatin- and SNAREopathies as examples of severe genetic neurodevelopmental disorders with an unmet need for rational interventions.
An assessment of the moral value of neuronal cell models and brain organoids
Sietske A.L. van Till, Mariia V. Maksimov, Ghislaine J.M.W. van Thiel, Eline M. Bunnik
Molecular Psychology: Brain, Behavior, and Society 2023, 2:15
Advances in stem cell technology enable neuroscientists to develop induced pluripotent stem cell (iPSC)-based neuronal models of varying complexity, ranging from single human brain cells to two-dimensional neuronal cell models and three-dimensional brain organoids. While the discussion on the moral status of brain organoids is taking center stage in the bioethical literature and is invariably linked to the presumed capacity of future brain organoids to develop some form of consciousness, analyses of the moral status of other – less complex – iPSC-based neuronal models are lacking. In this paper we aim to clarify the moral value of various types of existing neuronal models, including brain organoids. We show how it is made up of several layers that may encompass various sorts of considerations, including moral values, the results of empirical research, and biological characteristics. We identify four such layers – instrumental, intrinsic, symbolic, and relational – that are relevant for the assessment of the moral value of neuronal models. We demonstrate that it lies not in a capacity to develop some form of consciousness (which is absent in current iPSC-based neuronal models, including brain organoids), but in other considerations, including the genetic links between models and donors, the ability of models to mimic brain (dys)function, and their symbolic value, all of which are often overlooked in the bioethical literature. Also, we demonstrate that the ’thickness’ of the layers (i.e., their moral weight) increases when the neuronal model is more complex. Finally, we discuss the practical-ethical implications of our analysis for the use of neuronal models in research settings, for instance in relation to informed consent and biobank governance. Our four-layer framework can be applied also in moral assessments of other iPSC-based models, including emerging and future cell models.
Symbolic Value of Brain Organoids: Shifting the Focus from Consciousness to Sociocultural Perspectives on Resemblance
Sietske A L van Till, Eline M Bunnik
AJOB Neurosci. 2023 Apr-Jun;14(2):210-212. doi: 10.1080/21507740.2023.2188307
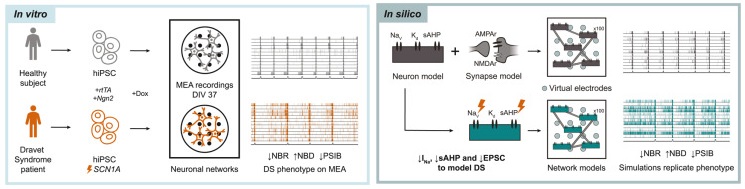
An in silico and in vitro human neuronal network model reveals cellular mechanisms beyond NaV1.1 underlying Dravet syndrome
Nina Doorn, Eline J H van Hugte, Ummi Ciptasari, Annika Mordelt, Hil G E Meijer, Dirk Schubert, Monica Frega, Nael Nadif Kasri, Michel J A M van Putten
Stem Cell Reports. 2023 Aug 8;18(8):1686-1700.
Human induced pluripotent stem cell (hiPSC)-derived neuronal networks on multi-electrode arrays (MEAs) provide a unique phenotyping tool to study neurological disorders. However, it is difficult to infer cellular mechanisms underlying these phenotypes. Computational modeling can utilize the rich dataset generated by MEAs, and advance understanding of disease mechanisms. However, existing models lack biophysical detail, or validation and calibration to relevant experimental data. We developed a biophysical in silico model that accurately simulates healthy neuronal networks on MEAs. To demonstrate the potential of our model, we studied neuronal networks derived from a Dravet syndrome (DS) patient with a missense mutation in SCN1A, encoding sodium channel NaV1.1. Our in silico model revealed that sodium channel dysfunctions were insufficient to replicate the in vitro DS phenotype, and predicted decreased slow afterhyperpolarization and synaptic strengths. We verified these changes in DS patient-derived neurons, demonstrating the utility of our in silico model to predict disease mechanisms.
Growth, body composition, and endocrine-metabolic profiles of individuals with Kleefstra syndrome provide directions for clinical management and translational studies
Arianne Bouman, Joyce M Geelen, Joost Kummeling, Annette Schenck, Yvonne G van der Zwan, Willemijn M Klein, Tjitske Kleefstra
Am J Med Genet A. 2023 Dec 29.
Mendelian neurodevelopmental disorders caused by variants in genes encoding chromatin modification can be categorized as Mendelian disorders of the epigenetic machinery (MDEMs). These disorders have significant overlap in molecular pathways and phenotypes including intellectual disability, short stature, and obesity. Among the MDEMs is Kleefstra syndrome (KLFS), which is caused by haploinsufficiency of EHMT1. Preclinical studies have identified metabolic dysregulation and obesity in KLFS models, but proper clinical translation lacks. In this study, we aim to delineate growth, body composition, and endocrine-metabolic characteristics in a total of 62 individuals with KLFS. Our results revealed a high prevalence of childhood-onset overweight/obesity (60%; 28/47) with disproportionately high body fat percentage, which aligns perfectly with previous preclinical studies. Short stature was common (33%), likely due to advanced skeletal maturation. Endocrine-metabolic investigations showed thyroid dysregulation (22%; 9/41), elevated triglycerides, and decreased blood ammonia levels. Moreover, hand radiographs identified decreased bone mineralization (57%; 8/14) and negative ulnar variance (71%; 10/14). Our findings indicate a high (cardio)metabolic risk in KLFS. Therefore, we recommend monitoring of weight and endocrine-metabolic profile. Supporting a healthy lifestyle and screening of bone mineralization is advised. Our comprehensive results support translational research and contribute to a better understanding of MDEM-associated phenotypes.
The End of Personification: The Mereological Fallacy in Science Communication on Brain Organoids
Sietske A L van Till, Eline M Bunnik
THE AMERICAN JOURNAL OF BIOETHICS 2024, VOL. 24, NO. 1, 51–54
In the last two decades, stem cell-based brain organoids have been developed to study disease mechanisms in various neurological, psychiatric, and developmental disorders. Simultaneously, there have been discussions in the bioethical literature on the ‘moral status’ of these models, which center mainly on their presumed potential to develop some form of consciousness (De Jongh et al. Citation2022). In her Target Article, Jennifer Blumenthal-Barby (Citation2024) argues that in discussions on the use of new technological entities, including chimeras, uploaded minds, artificial intelligence, and also brain organoids, bioethicists should stop using the concept of personhood, because it is unhelpful in the attempt to determine how these entities should be treated in practice. Instead, bioethicists should use other concepts, such as ‘interests’ or ‘sentience’, to determine whether new technological entities can be wronged, and ‘respect’, to determine what they are owed. In her article, Blumenthal-Barby mentions brain organoids only in passing. In this commentary, we apply and extend her argument to brain organoids and other neuronal cell models, including two-dimensional neuronal networks. We demonstrate that also in discussions on how brain organoids and other neuronal cell models ought to be treated, the use of the concept of personhood is a ‘problematic shortcut’. In addition, we argue that implicit personifications of brain organoids and other neuronal cell models, e.g., by comparing or equating these models with human beings, can likewise feed into unjustified assumptions about their moral status. We expose these implicit personifications as instances of the so-called ‘mereological fallacy’—i.e., the tendency to attribute human-like properties to the brain,—and show that they are omni-present in science communication on brain organoids. We warn the research community against the effects of this fallacy, and offer recommendations for science communication on brain organoid research.
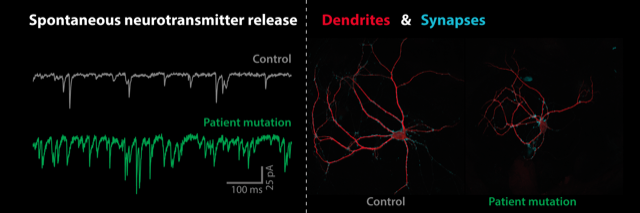
A de novo missense mutation in synaptotagmin-1 associated with neurodevelopmental disorder desynchronizes neurotransmitter release
Maaike A van Boven, Marta Mestroni, Petra J G Zwijnenburg, Matthijs Verhage, L Niels Cornelisse
Mol Psychiatry. 2024 Feb 6.
Synaptotagmin-1 (Syt1) is a presynaptic calcium sensor with two calcium binding domains, C2A and C2B, that triggers action potential-induced synchronous neurotransmitter release, while suppressing asynchronous and spontaneous release. We identified a de novo missense mutation (P401L) in the C2B domain in a patient with developmental delay and autistic symptoms. Expressing the orthologous mouse mutant (P400L) in cultured Syt1 null mutant neurons revealed a reduction in dendrite outgrowth with a proportional reduction in synapses. This was not observed in single Syt1PL-rescued neurons that received normal synaptic input when cultured in a control network. Patch-clamp recordings showed that spontaneous miniature release events per synapse were increased more than 500% in Syt1PL-rescued neurons, even beyond the increased rates in Syt1 KO neurons. Furthermore, action potential-induced asynchronous release was increased more than 100%, while synchronous release was unaffected. A similar shift to more asynchronous release was observed during train stimulations. These cellular phenotypes were also observed when Syt1PL was overexpressed in wild type neurons. Our findings show that Syt1PL desynchronizes neurotransmission by increasing the readily releasable pool for asynchronous release and reducing the suppression of spontaneous and asynchronous release. Neurons respond to this by shortening their dendrites, possibly to counteract the increased synaptic input. Syt1PL acts in a dominant-negative manner supporting a causative role for the mutation in the heterozygous patient. We propose that the substitution of a rigid proline to a more flexible leucine at the bottom of the C2B domain impairs clamping of release by interfering with Syt1’s primary interface with the SNARE complex. This is a novel cellular phenotype, distinct from what was previously found for other SYT1 disease variants, and points to a role for spontaneous and asynchronous release in SYT1-associated neurodevelopmental disorder.